Key Takeaways
- According to new research, life sciences leaders view AI as a solution to macroeconomic pressures and volatility, particularly in compliance, clinical trials, and healthcare professional engagement.
- The majority of life sciences leaders expect AI agents to help scale organizational capacity, strengthen operations, and improve sales and marketing engagement.
- AI adoption barriers remain, however, and include lack of trusted data, compliance risks, and lack of organizational change management plans.
Life sciences leaders are turning to AI and AI agents to combat increasing industry disruption. This comes as the industry faces new regulatory demands stretching compliance teams, increasingly complex clinical trials, and rising expectations from healthcare professionals (HCPs). A new study from Salesforce shows that life sciences leaders view AI as a powerful tool for navigating this landscape, with 94% expecting AI agents to be critical for scaling organizational capacity and strengthening operations. The research also highlights three key areas where AI can help stabilize the industry: compliance, clinical trials, and HCP engagement. In fact, 96% of leaders surveyed believe that AI agents will be “essential” within two years.
By the numbers:
Spotlight on AI in compliance: Life science leaders’ number one AI hurdle is also considered its most valuable use case
Expanding regulations and intensifying audits are hitting life sciences compliance teams especially hard.
- 64% of life sciences leaders flagged compliance team workloads as “heavily impacted” by recent volatility.
Regulatory and compliance leaders are eager to implement AI into their work, despite compliance’s reputation as a more risk-averse function.
- 64% of regulatory and compliance leaders within life sciences are “very excited” about using AI in their everyday work.
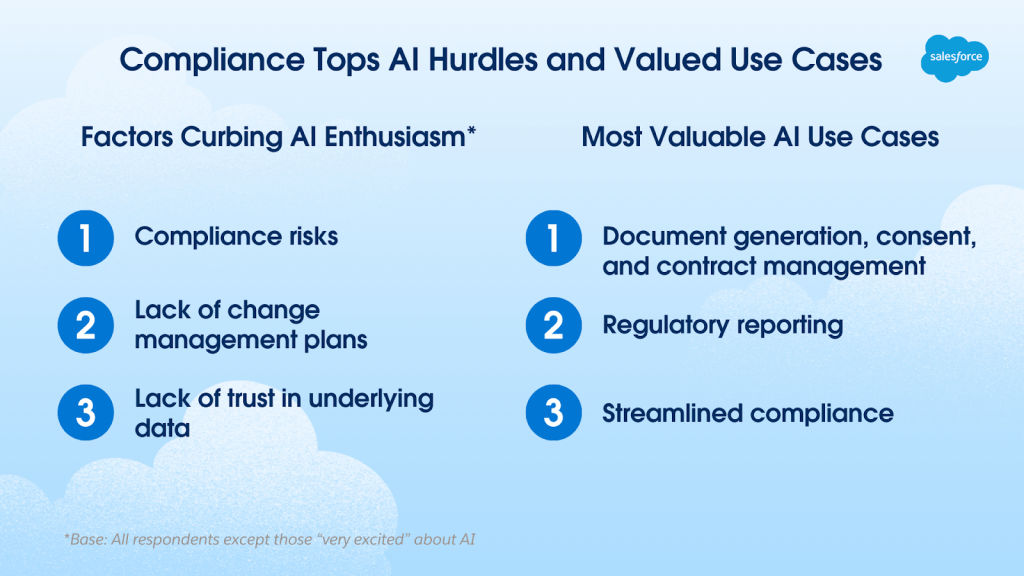
Paradoxically, compliance is both the top factor dampening AI enthusiasm in life sciences and the focus of its three most valuable use cases.
- Factors curbing excitement about using AI at work
- Compliance risks
- Lack of change management plans
- Lack of trust in underlying data
Base: All respondents except those “very excited” about using AI in daily work
- Most valuable AI use cases
- Document generation, consent, and contract management
- Regulatory reporting
- Streamlined compliance
Base: All respondents
AI is seen as a way to reduce workloads on routine tasks, raise compliance standards, and help compliance teams keep up with changing regulations. In fact, 94% of life sciences leaders say agents will be critical in keeping up with changing regulations.
“Agentic AI will simplify regulatory and compliance tasks, reduce staff stress, and keep us audit-ready,” said one Brazil-based VP of manufacturing, supply chain, and quality at a medical device company.
“We are using artificial intelligence to continuously interpret standard operating procedure (SOP) and quality assurance (QA) data, which is raising compliance standards,” said a Mexico-based director of manufacturing, supply chain, and quality at a pharmaceutical company.
The key takeaway: Leaders see compliance as an unexpected proving ground for AI in life sciences.
“The lesson, perhaps, is less about compliance itself and more about what it signals,” said Kyrsten Musich, Go-to-Market Strategy Leader of Healthcare & Life Sciences at Salesforce. “By focusing on compliance — a space sensitive to volatility yet an area where improvements can be easier to scale — executives are testing whether AI can relieve strain without adding risk. If it works here, the case for broader deployment across research and development (R&D) and commercialization will be far stronger.”
Spotlight on AI in clinical trials: Evolving trial requirements are reshaping how life sciences leaders plan, run, and scale innovation
Clinical trials remain the costliest step in therapy development, with each month of delay potentially costing millions. Trials are also experiencing significant disruptions from market swings, policy changes, and supply chain issues.
- Over half of life sciences leaders (57%) admit that trials are being majorly disrupted by market swings, policy changes, and supply chains, compounding well-known roadblocks like manual workflows and challenges tracking long-term outcomes.
- Top barriers to meeting new trial requirements
- Manual regulatory workflows (55%)
- Difficulty tracking long-term outcomes (38%)
- Disconnected R&D and clinical operations (25%)
- Site onboarding delays (25%)
- Recruitment and retention struggles (24%)
- Top barriers to meeting new trial requirements
In response, life sciences leaders are revisiting their innovation processes, with AI playing a key role.
- 94% say evolving trial requirements are reshaping how they plan, run, and scale innovation.
- R&D leaders are the biggest AI enthusiasts surveyed, with 81% being “very excited” about using AI in their everyday work.
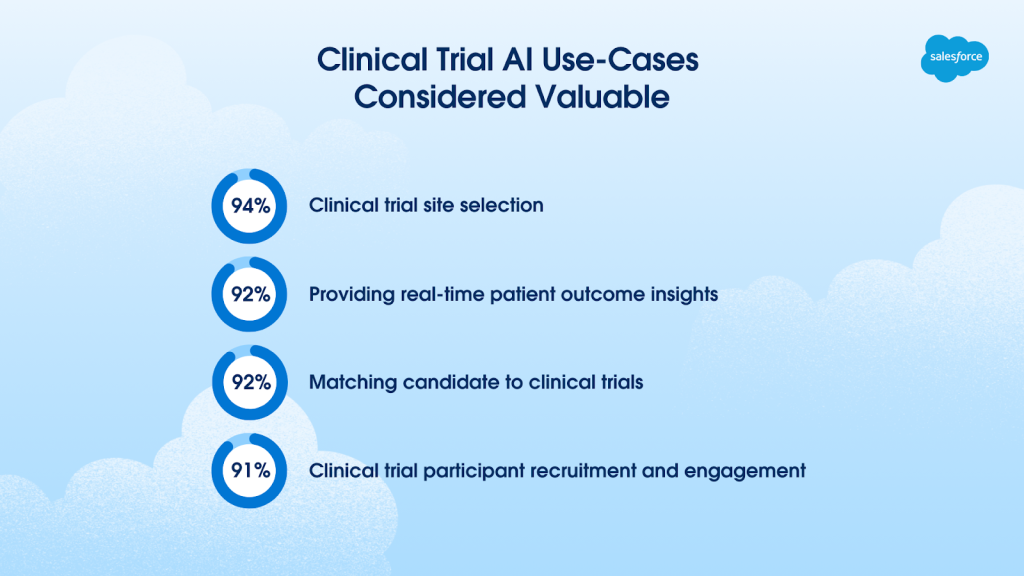
- Over 9 in 10 life sciences leaders consider AI in clinical trials a valuable solution. Examples of the top clinical trial AI use cases considered valuable:
- Clinical trial site selection (94%)
- Providing real-time patient outcome insights (92%)
- Matching candidate to clinical trials (92%)
- Clinical trial participant recruitment and engagement (91%)
Leaders believe AI will improve trial performance and accelerate lengthy development cycles.
“AI technology is expected to improve our clinical trial performance by enhancing qualified patients’ enrollment, monitoring multiple sites simultaneously, and conducting final data analysis in a very accurate and meaningful manner,” said a U.S.-based VP of R&D at a pharmaceutical company.
“Automating or semi-automating everyday, repetitive tasks may well free up resources and improve motivation to enable/direct resources to other more challenging aspects/areas. For example … agentic AI could autonomously coordinate literature reviews, preprocess raw data, or triage patient cohorts, freeing our most skilled scientists to focus on higher-order modeling, regulatory strategy, and clinical insight generation. I believe this shift would strongly accelerate cycles and streamline collaboration,” said a UK-based director of R&D at a pharmaceutical company.
Spotlight on AI in healthcare professional engagement: Companies spend billions on HCP engagement, yet more than a third of leaders say their strategies are broken
Leaders also see AI as a powerful tool to fix a costly problem in the commercial space: generic message overload that leaves HCPs tuning out.
- In the U.S. alone, healthcare and pharmaceutical companies spent upward of $30 billion on ads in 2024.
- Over one-third (37%) of life sciences leaders say their HCP engagement strategies, which include sales and marketing messages, are broken.
- 31% admit their sales and marketing teams aren’t scaling at the same pace as their product launches.
Weaker segmentation strategies may be to blame.
- Commercial leaders estimate 30% of their sales and marketing efforts are wasted — split between targeting the wrong people (15%) or sending the wrong message (15%).
- While most (58%) consider their segmentation strategy advanced and based on multiple data points, only 4% would consider their outreach state of the art.
- Even this may be an overestimate: Only 62% consider patient population demographics, and less than two-fifths (39%) factor in digital behaviors like channel preferences or content consumption patterns — revealing significant room for improvement.
Commercial leaders estimate 30%of their sales and marketing efforts are wasted on the wrong targets or the wrong message.
AI can be a powerful support, particularly AI agents that summarize, streamline, and respond to HCP communications.
- 63% of commercial leaders report they are “very excited” at the prospect of using AI in their everyday work.
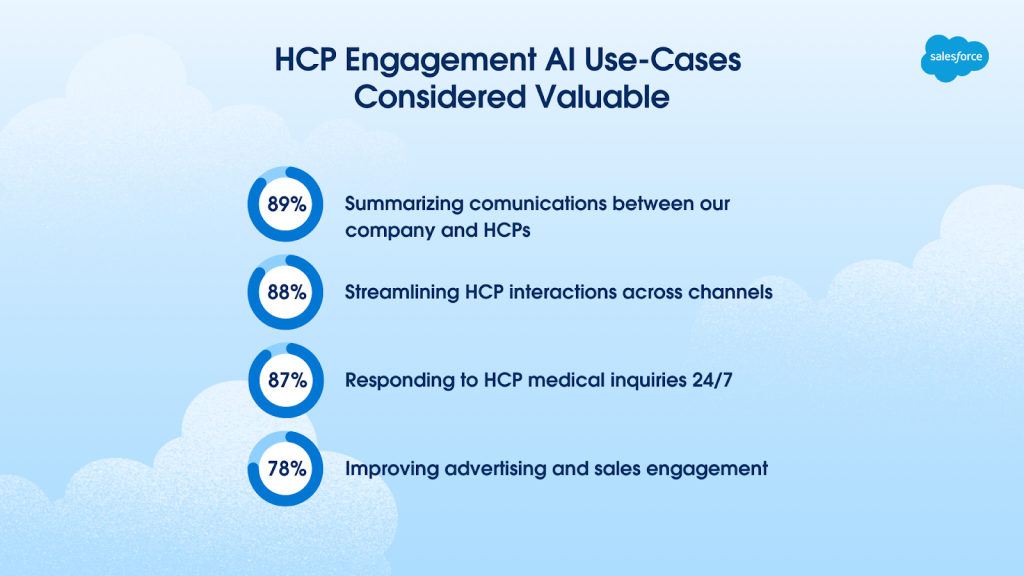
- HCP engagement AI use cases considered valuable include:
- Summarizing communications between companies and HCPs (89%)
- Streamlining HCP interactions across channels (88%)
- Responding to HCP medical inquires 24/7 (87%)
- Improving advertising and sales engagement (78%)
Leaders see AI agents as an answer to fragmented outreach.
- A German commercial executive sees agentic AI as a way to identify the right sales audience “in time and with precision.”
- A Mexico-based pharmaceutical executive describes it as a more dynamic tool, able to “track and segment our audience as their digital interactions evolve.”
“The life sciences industry spends billions on commercial outreach, but too often they’re shouting into the void. Every missed connection with a physician is wasted time and wasted trust. The problem isn’t effort — it’s fragmentation and volume of messages. Sales, marketing, and medical teams are working from disconnected systems, adding to the noise doctors already face,” explained Musich. “Agentic AI, powered by trusted data, can change that by unifying touchpoints and turning them into meaningful, timely conversations. The companies that embrace this shift will be the ones that truly break through.”
What’s holding life sciences companies back from further leveraging AI?
While 72% of life sciences leaders are “very excited” about AI, barriers to expansion remain.
- Top barriers to AI implementation or expansion include:
- Security, privacy, or compliance concerns
- Organizational change management
- Uncertainty around regulatory guidance on AI use
- Concern about using unproven or unfamiliar AI platforms
- Difficulty integrating AI into current tools and workflows
Trust in the underlying platform — and data — is a critical factor in earning life sciences workers’ trust in AI.
- Nearly all life sciences leaders agree that trusted data is essential for effective use of AI agents (97%) and that a widely used or proven platform is important for feeling confident using AI at work (96%).
- Yet in a separate survey, Salesforce found only 46% of life sciences technical leaders are fully confident that their data is consistently available, timely, and accurate.
To successfully leverage AI, leaders need the right foundation. “Life sciences leaders know that scaling AI requires more than just enthusiasm,” explained Musich. “They need a trusted foundation: timely, accurate, and auditable data anchored in a platform built for regulated industries.
That’s the promise of pairing Life Sciences Cloud with Agentforce, Salesforce’s trusted platform for building and deploying digital labor.
Kyrsten Musich, Go-to-Market Strategy Leader of Healthcare & Life Sciences at Salesforce
“That’s the promise of pairing Life Sciences Cloud with Agentforce, Salesforce’s trusted platform for building and deploying digital labor,” she continued. “It enables companies to confidently use AI for industry-specific needs like adverse event reporting or regulatory submissions, without compromising compliance. Now, leaders can do more than just stabilize operations during a period of uncertainty; they can reimagine their long-term strategies, accelerate cycles, and confidently navigate industry changes for years to come.”
Go deeper:
- Learn how Agent-first Life Sciences Cloud for customer engagement is transforming HCP engagement
- Register for Dreamforce to see all of the latest innovations for life sciences leaders
- Discover how Life Sciences Cloud drives healthier interactions with HCPs, partners, and patients
- See why customers like Takeda are choosing Salesforce Life Sciences Cloud to power their customer experience
- Find more insights on the Salesforce Stat Library
Methodology:
Data is sourced from a double-anonymous survey in partnership with RepData from July 23 to August 20, 2025. It included 400 life sciences leaders across research and development; regulatory and compliance; manufacturing, supply chain, and quality; commercial; and market access patient services. Respondents represented Brazil, Canada, France, Germany, Hong Kong, India, Japan, Mexico, Switzerland, United Kingdom, and the United States. This survey took place online.